
![]() Address:189 Guo Shou Jing Road Zhangjiang High-Tech Park,
Address:189 Guo Shou Jing Road Zhangjiang High-Tech Park,
Pudong, Shanghai, China
![]() Postcode: 201203
Postcode: 201203
![]() Telephone number:
Telephone number:
86-21-5080-1313
![]() Fax number: 86-21-5080-0721
Fax number: 86-21-5080-0721
![]() Email:center@mail.shcnc.ac.cn
Email:center@mail.shcnc.ac.cn
A collaborative study entitled “Two distinct domains of the glucagon-like peptide-1 receptor control peptide-mediated biased agonism” carried out by two research teams at Shanghai Institute of Materia Medica, Chinese Academy of Sciences/School of Pharmacy, Fudan University and Monash Institute of Pharmaceutical Sciences, Australia, was published by The Journal of Biological Chemistry on June 15, 2018 (http://www.jbc.org/content/293/24/9370.abstract).
G protein-coupled receptors (GPCRs) belong to a family of membrane proteins characteristic of seven transmembrane domains. Glucagon-like peptide-1 receptor is a member of the glucagon receptor subfamily of class B GPCR and has been implicated in the treatment of type 2 diabetes and obesity. A handful of therapeutic agents, namely, glucagon-like peptide-1 receptor agonists, such as Exenatide, Liraglutide, Dulaglutide, etc. are presently on the market with annual sales of billions of dollars. These drugs require different administration frequency and possess variable efficacies, adverse event as well as tolerance, which may be related to the outcome of biased agonism of glucagon-like peptide-1 receptor.
Biased agonism is a term describing the phenomenon that GPCRs are highly dynamic proteins and can adopt numerous ligand-specific conformational ensembles with distinct impact on signaling and regulatory profiles, even with ligands acting via a common binding pocket. The N-terminal and the residues on transmembrane loop 1 (TM1) near the extracellular domain of glucagon-like peptide-1 receptor are very flexible and usually missing in the three-dimensional crystal or Cryo-electron microscopy (cryo-EM) structures of the receptor resolved thus far. Their roles in the full-length wild-type receptor activation are not clear.
To overcome this hurdle, scientists let by Drs. Ming-Wei Wang, Patrick Sexton and Denise Wootten started the joint effort three years ago. Focusing on the N-terminal and TM1 region near the extracellular domain, they mutated 28 residues and used several techniques to systematically study receptor binding affinities and activation efficacies on different signaling pathways (cAMP, pERK1/2 and Ca2+) of several peptidic ligands. It was found that different residue groups were able to modulate both affinity and signaling in a varied but overlapping manner, and different ligands bind to the same receptor in different conformation thereby transducing distinct downstream signals. Clearly, the N-terminal and TM1 region near the extracellular domain of glucagon-like peptide-1 receptor play an important role in controlling peptide-mediated biased agonism. These findings provide molecular insights into the initiation of receptor activation and new direction in drug design by maximizing the physiological benefit of biased agonism.
This work was supported by National Health and Medical Research Council of Australia, the National Natural Science Foundation of China, Strategic Priority Research Program of Chinese Academy of Sciences and Shanghai Science and Technology Development Fund. The first author, Saifei Lei, received the Postgraduate Overseas Study Fellowship from Chinese Academy of Sciences when performing part of her research in Australia.
Link to the paper:
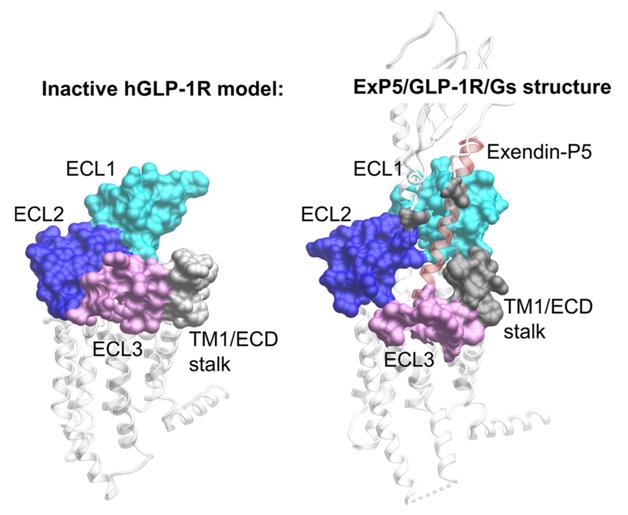
Inactive glucagon-like peptide-1 receptor model and active structure activated by Exendin-P5 in complex with Gs protein